On 11 December 2025, the
Council and the European Parliament reached agreement on the EU Pharma Law
Package, also known as the General Pharmaceutical Legislation. No text has been
published yet. The agreement will need to be endorsed by the Council of the European Union and the
European Parliament before being formally adopted and the final text is expected to be published in the next
few months before entry into
force upon publication in the EU's Official Journal.
The EU Pharma Law Package is a full recast of European pharmaceutical regulatory law and includes amendments in many areas, ranging from marketing authorisation processes, to manufacturing, pharmacy formulations/compounding, prevention of supply shortages, and to environmental risk assessment. Also, the Bolar exemption will be broadened, allowing activities of generics and biosimilars inter alia in the areas of pricing, reimbursement and procurement prior to patent expiry.
Much debated since the European Commission published its initial proposal in 2023, key changes relate to the shortening of (i) regulatory data protection for innovator medicinal products, and (ii) orphan market exclusivity for orphan medicinal products for rare diseases.
- Regulatory data protection will consist of 8 years of data exclusivity, same as under the current legal framework, and one additional year of market exclusivity, so a total of 8+1 years of protection, compared to 8+2 years in the current system. There will be a possibility to obtain one additional year of exclusivity (8+1+1) under certain circumstances and another year (8+1+1 or 8+1+1+1) for a new indication of significant clinical benefit, with a capped overall regulatory protection of 11 years. The provision as introduced by the Council in its proposal to facilitate access to medicines, providing Member States the possibility to require marketing authorisation holders to (in short) place a product on the market in that Member State is kept in the agreed text of the reform package. Under this new mechanism, if the marketing authorisation holder does not comply with such request of a Member State within 4 years after grant of the marketing authorisation, (prolongation of) market protection is not applicable in that Member State, allowing competitors to enter the market sooner. Following the press release of the Council of the EU, safeguards have been added to the text of the proposal clarifying obligations for companies and member states and preventing the use of this mechanism for parallel trade.
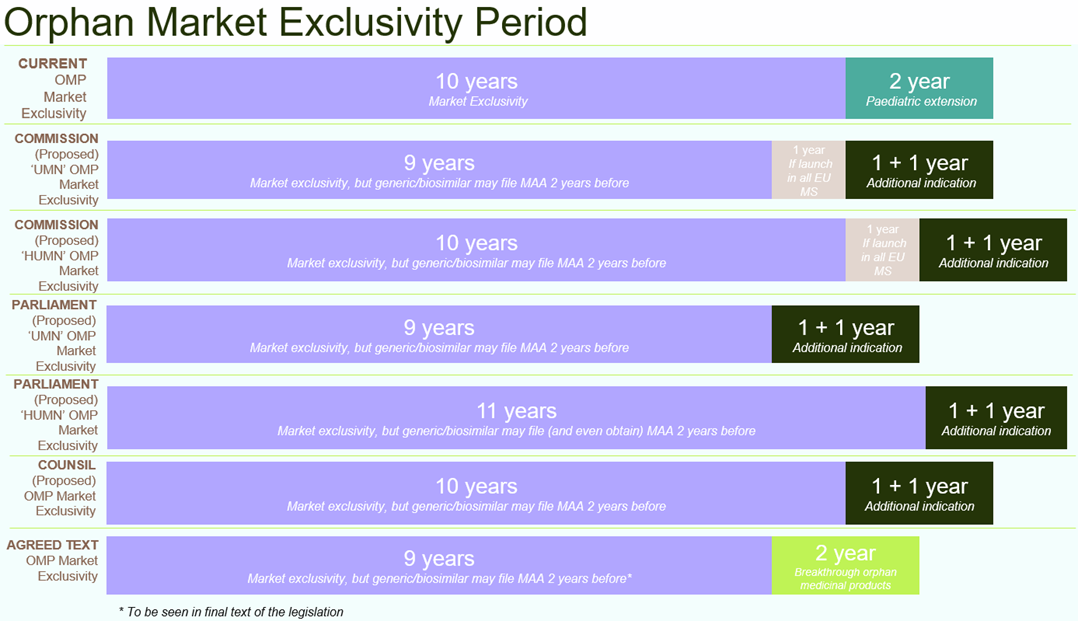
- Orphan market exclusivity will be reduced from the current 10 years to 9 years. Extension by another 2 years will be possible for “breakthrough orphan medicinal products”. Although the final text has not yet been published, previous drafts also included the concept of “global orphan marketing authorisation”, which would no longer grant additional separate orphan market exclusivity for second or further orphan therapeutic indications. Also, previous drafts allowed generics, biosimilars or other second applicants to apply for marketing authorisation two years before expiry of orphan market exclusivity thus effectively reducing the innovator’s market exclusivity compared to the current legal framework.
It is expected that the
final text will be endorsed and published in the next few months and that,
after a transition period, the new legislation will start to apply from
mid-2028.
Authored by Hein van den
Bos, Joerg Schickert, and Julia Mischie.

 Search
Search


 Search
Search






